Characterization of the biomolecular interactions and screening of bioactive compounds through Surface Plasmon Resonance (SPR)
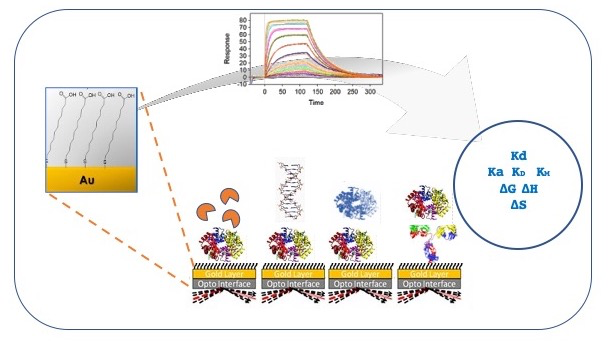
The activity of the SPR laboratory is focused on the characterization of the biomolecular interactions, which allow to measure the kinetic constants, the affinity and the stoichiometry of the binding. The high sensitivity of the instrumentation allows the analysis of the interactions involving small molecules, as well as the screening of compound libraries.
The SPR biosensor technology allows, through real-time and label-free experiments, the rapid characterization of molecular interactions (i.e. proteins, DNA, antibodies, peptides, vesicles) with high sensitivity and accuracy. The analysis provides high quality kinetics and affinity measurements, but can be also applied for thermodynamic and stochiometric studies, for quantitative determination and to perform antibody screening and identification.
The SPR facility of IC-CNR is equipped with a SensiQ Pioneer AE platform, a highly sensitive and fully automated instrument which provides accurate kinetics and affinity determination even with small molecules (<70 Da). For this reason, it can be used both for traditional applications and for screening of large compound libraries.
Our research activity, among many collaborations arising from different projects, aims to the identification of novel bioactive compounds (drug discovery) and to the study of the structure-activity relationship of biomolecules (nuclear receptors, membrane proteins, enzymes).
– Capelli D. et al. “Surface Plasmon Resonance as a tool for ligand binding investigation of engineered GPR17 receptor, a G protein coupled receptor involved in myelinization”. Front Chem 2020; 7:910.
– Montanari R. et al. “Screening of saponins and sapogenins from Medicago species as potential PPARγ agonists and X-ray structure of the complex PPARγ/caulophyllogenin”. Sci Rep 2016; 6:27658.
– Boi D. et al. “PHA-680626 Is an Effective Inhibitor of the Interaction between Aurora-A and N-Myc”. Int J Mol Sci 2021; 22(23):13122
– Spizzichino S. et al. “Cytosolic localization and in vitro assembly of human de novo thymidylate synthesis complex”. FEBS J 2021; 289(6):1625-1649


