Molecules and regulatory mechanisms of proteostasis in protein misfolding diseases
The alteration of proteostasis, i.e the control of the proteome integrity, is the basis of many diseases (eg cancer, Alzheimer’s, Parkinson’s and diabetes). The research activity is aimed at the design of small molecules capable of restoring cellular proteostasis.
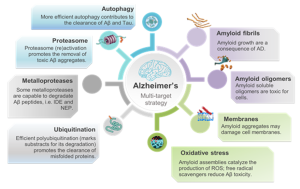
There is mounting urgency to find new drugs for the treatment of Protein Misfolding Diseases (PMDs) including Diabetes Mellitus Type II (T2DM), and Alzheimer’s diseases (AD). Based on the “amyloid hypothesis” much efforts have been devoted to design molecules able to halt disease progression by inhibiting amyloid growth. Unfortunately, all these attempts proved to be unsuccessful so far, suggesting that a more detailed knowledge of all the molecular events leading to cell damage is needed. It is known that cells express an orchestrated array of proteolytic machineries that control protein homeostasis (proteostasis) in a given range of adverse environmental conditions. This group conduct research at the cutting edge between biological and physical chemistry focusing on the design of small molecules (e.g. natural compounds, porphyrins, pyrazolones etc.) aimed at restoring the whole proteostasis network, rather than merely inhibit toxic protein aggregation. Our work spans fundamental science related to thermodynamics of protein stability and protein-protein interactions (PPis) through to applications in medicinal chemistry for the development of bioconjugates, involving chemically tailored interfaces between lipids, proteins, nucleic acids, small molecules and metal ions.
– D. Milardi, E. Gazit, S. E. Radford, Y. Xu, R. U. Gallardo, A. Caflisch, G. T. Westermark, P. Westermark, C. La Rosa, and A. Ramamoorthy, Proteostasis of Islet Amyloid Polypeptide: A Molecular Perspective of Risk Factors and Protective Strategies for Type II Diabetes, Chem. Rev. (2021), 121, 1845−1893.
– F. Bellia, V. Lanza, S. Garcıa-Vinuales, I. M. M. Ahmed, A. Pietropaolo, C. Iacobucci, G. Malgieri, G. D’Abrosca, R. Fattorusso, V. G. Nicoletti, D. Sbardella, G. R. Tundo, M. Coletta, L. Pirone, E. Pedone, D. Calcagno, G. Grasso and D. Milardi, Ubiquitin binds the amyloid b peptide and interferes with its clearance pathways, Chem. Sci. (2019), 10, 2732-2742.
– G. Malgieri, G. D’Abrosca, L. Pirone, A. Toto, M. Palmieri, L. Russo, M. F. M. Sciacca, R. Tatè, V. Sivo, I. Baglivo, R. Majewska, M. Coletta, P. V. Pedone, C. Isernia, M. De Stefano, S. Gianni, E. M. Pedone, D. Milardi* and R. Fattorusso*, Folding mechanisms steer the amyloid fibril formation propensity of highly homologous proteins, Chem. Sci., (2018), 9, 3290–3298.
– A. M. Santoro, A. Cunsolo, A. D’Urso, D. Sbardella, G. R. Tundo, C. Ciaccio, M. Coletta,* D. Diana, R. Fattorusso,* M. Persico, A. Di Dato, C. Fattorusso, D. Milardi* and R. Purrello*, Cationic porphyrins are tunable gatekeepers of the 20S proteasome, Chem. Sci., (2016), 7, 1286–1297.


